Abstract

Background: Allogeneic hematopoietic stem cell transplantation (HCT) offers the best chance of cure for children suffering from high-risk (HR) acute myeloid leukemia (AML). Over the last decade, a consensus between study groups has increasingly been developed on when and how to perform HCTs in these patients. However, the fate of children experiencing AML relapse after a first allogeneic HCT remains dismal. Methods: With the aim of analyzing the outcome of children that had documented AML relapse after a first allogeneic HCT, we performed a retrospective analysis collecting data from cooperative groups participating in the I-BFM consortium. A questionnaire was developed aiming to collect comparable data on children that did or did not proceed to a second allogeneic HCT. The questionnaire was sent to the national study coordinators and completed by the national groups from Australia, Austria, Belgium, Czech Republic, Denmark, Finland, Germany, Israel, Italy, the Netherlands, New Zealand, Norway, Poland, Sweden, and Switzerland. Overall survival (OS) and event-free survival (EFS) were estimated from the date of relapse after the first allograft or 2nd HCT to the date of an event or last follow-up. Probabilities of OS and EFS were calculated according to the Kaplan and Meier method. Results: 336 patients experiencing relapse between January 2005 and December 2016 were identified. 199 were male and 137 female. The median age was 8.6 years (range 0.4; 26.0). 178 (53%) were younger than 10 years and 138 (47%) 10 years or older. 156 (47.4%) had originally been transplanted in CR1, 148 in CR2 (45.0%), and 25 for refractory disease (7.6%). OS and EFS for the total group was 14% (standard error, SE=0.02) and 2% (SE=0.01) at 4 years respectively. Survival curves were superimposable for patients that had been primarily transplanted in CR 1 (OS 15%) and CR2 (OS 15%). Children originally transplanted in refractory disease had a significantly inferior OS of 4% (p=0.009) once relapsed after 1st HCT. OS was comparable for children presenting with either HR or SR molecular and cytogenetic features before HCT (p=0.85). A time interval between 1st HCT and relapse < 6 months was associated with poorer OS (7% vs 29% for patients relapsing > 6 months after the 1st allograft, p < 0.001).
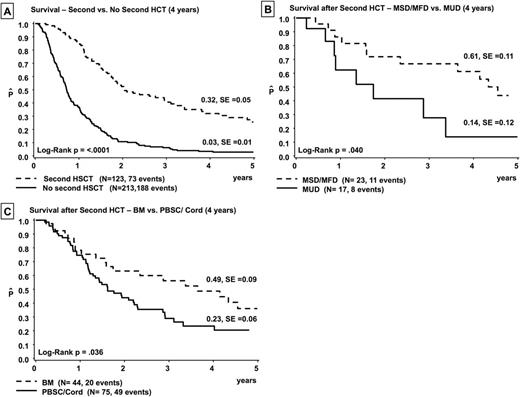
Of note, only 123 (36.6%) children proceeded to a 2nd HCT. Importantly, the 4-year OS probability was 32% for children receiving a second transplant. This compared favorably with a 4-year OS of only 3% for those children who did not receive a 2nd HCT (p < 0.0001, Fig. A). Disease progression and failure to achieve another remission were the main reasons (58.6%) for not proceeding to a 2nd HSCT whereas poor performance status was an exclusion criterion for 11 %. Treatment failure after 2nd HCT was disease-related in 65% of patients; transplant-related mortality (TRM) accounted for 21% of deaths. For the remaining 14% of children, data were not attributable.
Graft source (bone marrow vs. peripheral blood stem cells/ cord blood, 49% versus 23%, p= 0.036, Fig. B) and donor type (matched sibling/ family donor vs. matched unrelated donor, 61% versus 14%, p=0.04, Fig. C) influenced patient's outcome after the 2nd allograft. Children experiencing any type of graft-versus-host disease (GVHD) had a better, although not statistically significant, probability of OS as compared to those who did not develop GVHD (47 % vs. 25%).
Sauer: Neovii: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal